Ionizer™ Technology: It’s Simple, All You Need Is Air™
Unlimited, On-Demand Nitric Oxide From Hospital to Home



- Hospital NICU setting
- Low-concentration inhaled nitric oxide (iNO): ≤80 ppm
- To treat term and near-term neonates with hypoxic respiratory failure, commonly referred to as persistent pulmonary hypertension of the newborn (PPHN)
- No cylinders. No hassle.
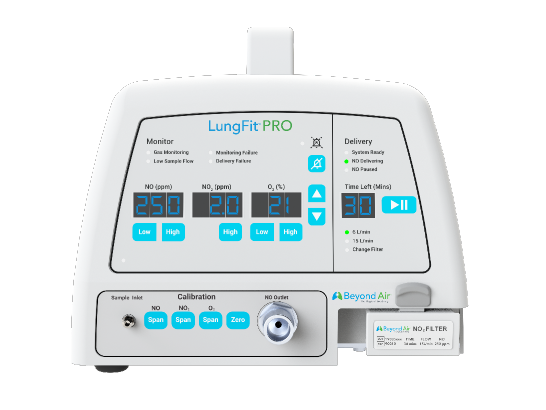
- Hospital setting
- High-concentration iNO: 80 to 400 ppm
- To treat community-acquired viral pneumonia (including COVID-19) and bronchiolitis
- No cylinders
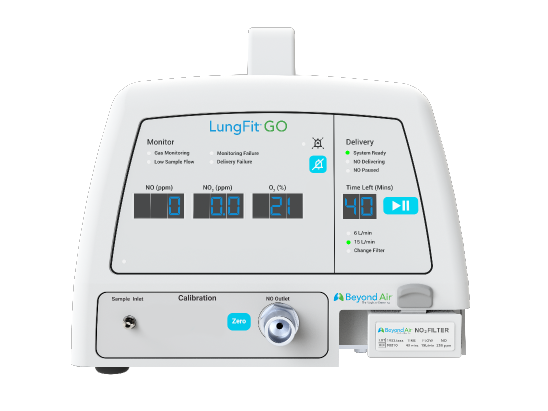
- At-home treatment
- High-concentration iNO: 80 to 400 ppm
- To treat nontuberculous mycobacteria (NTM) and lung infections in patients with underlying chronic obstructive pulmonary disease (COPD)
- No cylinders
LungFit: A Family of Products for Flexibility in iNO Dosing
| Download Spec Chart |  |
 |
 |
|---|---|---|---|
| Use location | Hospital NICU setting | Hospital setting | At-home treatment |
| For treatment of | Term and near-term neonates with hypoxic respiratory failure, commonly referred to as persistent pulmonary hypertension of the newborn (PPHN) | Community-acquired viral pneumonia (including COVID-19) and bronchiolitis | Nontuberculous mycobacteria (NTM) lung infection and in patients with underlying chronic obstructive pulmonary disease (COPD), suffering from persistent lung infections |
| iNO concentration | Low-concentration iNO: ≤80 ppm | High-concentration iNO: 80 to 400 ppm | High-concentration iNO: 80 to 400 ppm |
| Supplemental oxygen can be utilized through the system |
|
|
– |
| Alarms to monitor performance |
|
|
|
| Disposable NO2 Smart Filters |
|
|
|
| System weight | ~44 lb | ~20 lb | ~18 lb |
| Usable with standard 110V or 220V electric outlets |
|
|
|
| Visit Product Page | See Active Pipeline | ||
A Future Built on a Legacy of Innovation
The LungFit family of products are designed by the same inventors who developed the first nitric oxide delivery system and other systems in use worldwide.*
The Benefits of the First-in-Class LungFit Technology
-
 Transforms iNO treatment with simplicity and certainty to provide effective and efficient patient care
Transforms iNO treatment with simplicity and certainty to provide effective and efficient patient care -
 Improves operating economics for the hospital by avoiding cost and logistics associated with 45-lb high-pressure cylinder iNO treatment
Improves operating economics for the hospital by avoiding cost and logistics associated with 45-lb high-pressure cylinder iNO treatment -
 Reduces burdensome training, inventory, and storage requirements by eliminating the need for iNO cylinders
Reduces burdensome training, inventory, and storage requirements by eliminating the need for iNO cylinders
-
 Convenient storage of small NO2 Smart Filters and disposable accessories at the point of care
Convenient storage of small NO2 Smart Filters and disposable accessories at the point of care -
 Enables integration of iNO management into hospital point-of-use solutions for streamlined logistics
Enables integration of iNO management into hospital point-of-use solutions for streamlined logistics -
 Reduces risk of NO2 exposure to patient and clinician with NO2 Smart Filter and integrated autopurging1
Reduces risk of NO2 exposure to patient and clinician with NO2 Smart Filter and integrated autopurging1
Product News and Investor
Relations Announcements
Revolutionizing Nitric
Oxide Therapy
Reference: 1. Rimkus M, Montgomery F, Bathe D. On-demand nitric oxide for ventilator-based nitric oxide inhalation: a risk-reduction perspective. Abstract presented at: 22nd Congress of the International Society for Aerosols in Medicine (ISAM); May 24, 2019; Montreux, Switzerland. Accessed May 13, 2022.
*The inventors of the LungFit Systems are also listed as inventors on the patents for the following nitric oxide delivery systems: INOvent, INOmax DSIR, INOmax DSIR Plus, INOmax DSIR Plus MRI.
Caution: LungFit®PRO and LungFit®GO are investigational devices, limited by federal (or United States) law to investigational use.

